About Us
About CardioReGenix
The World Health Organization (WHO) estimates 17.7 million people die each year from cardiovascular disease (CVD), an estimated 31% of all deaths worldwide. While progress with conventional treatments is making incremental gains, there remains a need to develop innovative therapeutic approaches. Gene therapy has gained significant momentum, mainly for treatment of rare monogenetic diseases, but marketing authorization for gene therapy products has not impacted diseases such as CVD.
Recent deeper understanding of the molecular/cellular mechanisms of CVD, and technology associated with more efficient and safer gene therapy vectors, has allowed new opportunities for development of next generation advanced therapy medicinal products (ATMPs) for CVD.
CardioReGenix focuses on technological innovations for the treatment of CVD, in particular heart failure and myocardial ischemia. We aim to overcome the existing bottlenecks in CVD gene therapy by:
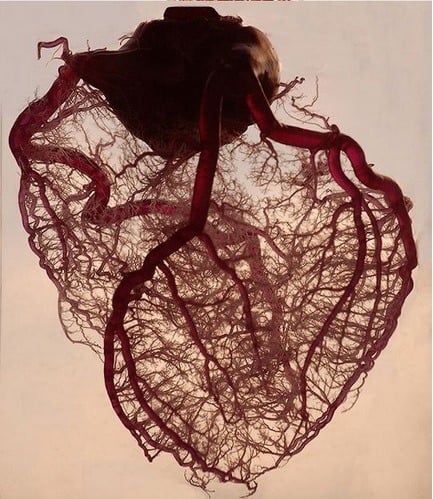



Supported By Robust Preliminary Data
The CardioReGenix project is supported by robust preliminary data and a multi-disciplinary consortium harnessing know-how in vector development, GMP manufacturing, preclinical and clinical cardiology, regulation of ATMP and liaising with EMA, business development and clinical translation. The CardioReGenix project will strengthen Europe's competitive position in gene therapy development for CVD and improve the prospect of a gene therapy successfully treating patients suffering from CVD.